Flow Chemistry in Organic Synthesis:
A powerful tool for development of synthesis and industrial production
Introduction
The origins of flow chemistry can be traced to the early 20th century, the petroleum sector was a pioneer in adopting continuous flow reactors, enabling more efficient and controlled refining of crude oil. By the mid-20th century, the principles of flow chemistry had permeated other sectors, including pharmaceuticals, fine chemicals, and materials science. Today, flow chemistry development is driven by the need for processes that are not only scalable and reproducible but also the need for rapid optimization, precise parameter control and greater inherent safety and environmental sustainability.
The paradigm shift from batch to continuous processing has profoundly influenced the evolution of chemical synthesis over the past century. Among the most significant advancements in this domain, flow chemistry—or continuous flow chemistry—has emerged as a transformative methodology, offering substantial improvements in efficiency, safety, and sustainability[1]. This approach, grounded in decades of industrial and academic research, represents a cornerstone of modern chemical engineering and process optimization.
In this article, we explore the potential of flow chemistry on organic synthesis, highlighting recent progress and applications, as well as its advantages over batch methods. Professor François-Xavier Felpin (Nantes Université, France) shares insights into the field and his perspective on the future development and application of this technique.
Finally, a case study of flow chemistry experiment conducted by Oxeltis scientists will illustrate the everyday applications of this powerful approach and the exciting possibilities it offers.
- Flow Chemistry: Powerful and versatile tool for research and scale-up
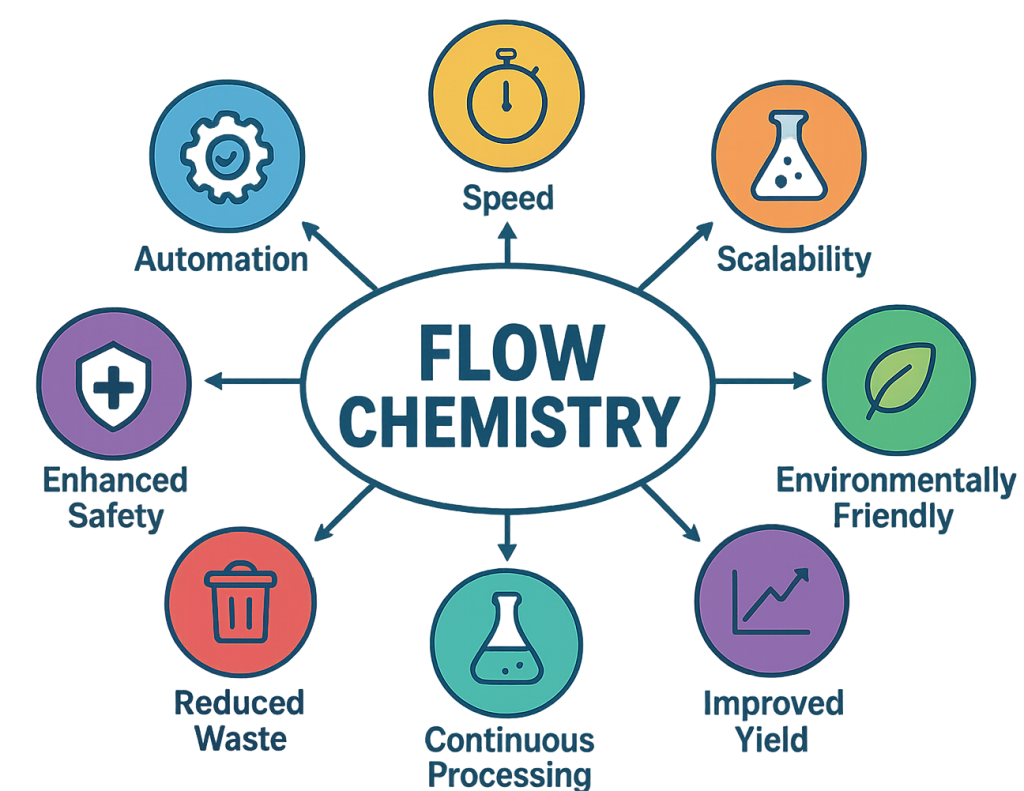
Flow chemistry goes far beyond being an alternative to batch processing: it integrates complementary techniques such as photochemistry[2], electrochemistry[3], heterogeneous catalysis[4],[5], and even high-throughput experimentation (HTE)[6], making it a powerful platform for tackling a wide range of synthetic challenges. Flow chemistry also permits multistep synthesis[7] and reactive intermediates which are directly used.[8] Moreover, the continuous nature of the process facilitates real-time monitoring[9] and optimization, paving the way for smarter, more sustainable manufacturing.
One of the key features of flow chemistry is the fast heat transfer due to the small size of the reactor volume, whether during heating or cooling. This advantage reduces the risks of thermal degradation of reagents or products and consequently improves yield. Additionally, continuous flow systems enable high-pressure and high-temperature reactions that would be unsafe in large batch vessels. Furthermore, with optimized residence time, products are removed from the reaction site as they are produced, thus preventing over functionalization or overreaction.
The safety of this technique is also worth highlighting. Small quantities of reactants are present in the reactor at any given time, significantly reducing the risk of runaway reactions. Flow chemistry also mitigates the operational risks associated with handling hazardous or explosive reagents and enclosed systems, thereby enhancing both personnel and facilities safety.
However, one of the most important advantages of flow chemistry is the ease of scaling-up research-lab experiments to industrial processes with ensured reproducibility. Flow chemistry allows « scale-out » rather than « scale-up »: the same reactor design can be replicated in parallel to achieve larger production volumes while maintaining identical reaction conditions. This attribute is particularly appealing in pharmaceutical contexts where regulatory reproducibility is crucial. Flow chemistry drastically reduces the need for extensive pilot scale optimization.

- Expert Insights: Interview with Prof. François-Xavier Felpin
François-Xavier Felpin, born in France in 1977, is a Professor at Nantes Université (France). After earning his PhD from Nantes Université in 2003, he pursued postdoctoral studies at Ohio State University (USA). In 2004 he joined the University of Bordeaux as an Assistant Professor. In fall 2011 he moved to the University of Nantes, where he was promoted to full Professor. In the last ten years his team contributed to the development of autonomous flow chemistry and algorithmic approaches for the optimization of chemical reactions. He has co-authored more than 140 publications and is the co-inventor of 10 patents and one piece of software.
- In your opinion, what are the main advantages of flow chemistry compared to traditional batch processes at the lab scale?
To begin with, I must say, to be perfectly honest, that flow chemistry is even more relevant in development and production than in R&D. However, even at the laboratory scale, where economic and environmental constraints are less pressing, flow chemistry remains a particularly interesting and complementary technology to batch processes. Flow chemistry allows for much better control of heat transfer, leading to significantly improved temperature regulation. The consequences are numerous: greater reproducibility, enhanced kinetics, better control of selectivities, and increased safety. Moreover, I often say that the use of high temperatures combined with a back-pressure regulator offers the advantages of a microwave reactor without its drawbacks. Finally, flow chemistry proves to be far more reproducible in photochemistry thanks to the use of narrow-diameter channels.
- Could you share practical considerations for reproducibility and scaling from milligrams to grams?
When scaling up from the milligram to the gram scale, there are several possible approaches, but to ensure the best reproducibility the simplest option is to keep the same equipment whenever possible. Increasing the operating time or duplicating the units (numbering up), provided that sufficient equipment is available, makes it possible to achieve excellent reproducibility. Another option is to increase the size of the reactor without changing the other components of the experimental setup, except for the flow rate. Several manufacturers offer reactors in different sizes that make this approach feasible.
- What are the main limitations or challenges that still restrict broader adoption?
Continuous synthesis is not universal or a magic recipe, and I must even admit that it is less so than batch chemistry, which benefits from a much longer history. The first barrier to the adoption of flow chemistry, whether in R&D or in development, is the lack of expertise within teams and a certain degree of conservatism. However, mindsets are changing rapidly, and this obstacle is gradually disappearing. The second barrier concerns equipment. Few laboratories or industries are currently equipped, as the initial investment is relatively high. While this investment can be quickly amortized in production, this is less true in R&D, where return on investment is not a relevant metric—particularly for academic laboratories. Finally, the last limitation lies in the non-universal nature of continuous synthesis itself. Reactions with very long kinetics, heterogeneous media, and reagents that are too aggressive for the equipment represent significant constraints that must be kept in mind.
- What role does inline analysis (IR, NMR, MS) play in flow chemistry at the discovery scale?
In-line/online analysis, regardless of the analytical tool used, plays an important role in the real-time and/or in-situ monitoring of chemical transformations. This makes it possible to rapidly adjust experimental conditions or to identify short-lived intermediates. Analytical monitoring is also an essential element for introducing a degree of automation. All of this holds true both in R&D and at the industrial scale.
- For a medicinal chemistry group, what would be an ideal “starter kit” of flow chemistry tools?
It is always difficult to answer this question, as several approaches coexist. If your work does not involve any consideration of scale-up, the use of syringe pumps is a cost-effective option for setting up experimental systems. On the other hand, if you are looking for greater flexibility, piston pumps are a relevant choice. Still, you can buy two or three syringe pumps for the price of a good piston pump! But flow chemistry is not limited to the pumping system. It is essential, of course, but not the only component. You will also need tubing—HPLC tubing is perfectly suitable for this purpose—along with fittings and reactors. Tubular reactors are the least expensive option and are well adapted. Here again, the use of HPLC consumables is highly recommended. And then, if you become hooked, you will need to consider acquiring inline analysis, a sample-handling robot… but that is another story.
- How do you see the practical application of algorithmic approaches for the optimization of chemical reactions and how does your research apply to autonomous flow chemistry?
My group has been an active player in the field of optimization and autonomous flow chemistry ultimately leading to the emergence of self-optimizing flow reactors[10]; driven by the demand for faster reaction optimizations and the imperative to reduce the development costs of synthesized products. These robotic flow platforms utilize automated reactors, online analytical tools[11], and feedback algorithms that learn from previous experiments, enabling them to select future experiments aimed at optimizing the chemical transformation under study (see Figure 1). My group works at the interface of computer sciences, chemical engineering, organic synthesis and analytical chemistry. These last years, the team has developed new optimization algorithms, ultra-fast NMR analytical techniques[12],[13], and orchestration softwares for device control.
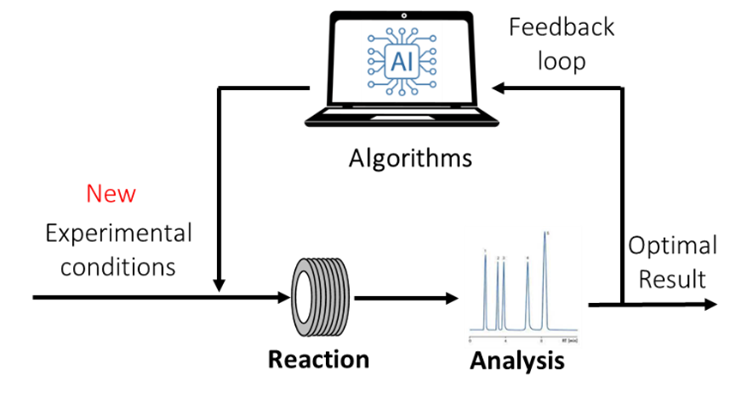

Figure 1. (Left) scheme of the feedback loop for autonomous AI driven optimization. (Right) Prof. Felpin’s set-up for autonomous flow optimization.
- Applications of Flow Chemistry at Oxeltis
Oxeltis follows Seeberger’s well-known decision-making guide (from “The Hitchhiker’s Guide to Flow Chemistry”)[14] to assess whether using a flow chemistry setup is relevant for each project. This systematic approach ensures that flow chemistry is implemented when it offers clear technical, time-saving or economic advantages. By doing so, we optimize resource use while maintaining flexibility in our synthetic development strategies.
Oxeltis has been using flow chemistry for the past three years. During the development of a complex multistep synthesis performed in batch, yield limitations in the first step prompted our team to explore alternative synthetic approaches. One of the key challenges involved the 2′-chlorination of a 2′-fluorolactone with N-chlorosuccinimide (NCS) at –78 °C (Scheme 1). Although this transformation is well described in the literature and had previously given satisfactory yields at Oxeltis on a small scale, we encountered significant difficulties when attempting to scale it up.

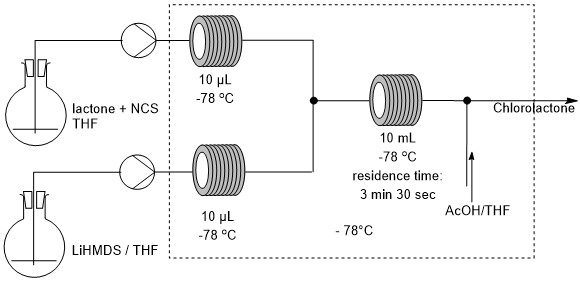
Figure 2. (Left) scheme of the flow chemistry set-up. (Right) picture of the flow chemistry set-up.
In the standard batch procedure, a solution of lithium bis(trimethylsilyl)amide (LiHMDS) is added dropwise at low temperature to a mixture of the lactone and NCS under strictly anhydrous conditions. However, the resulting α-chloro-fluorolactone is highly sensitive to bases, which promotes the formation of undesired side products. Upon scale-up, the prolonged addition time of the base solution further exacerbates this issue, leading to reduced yields and poor reproducibility.
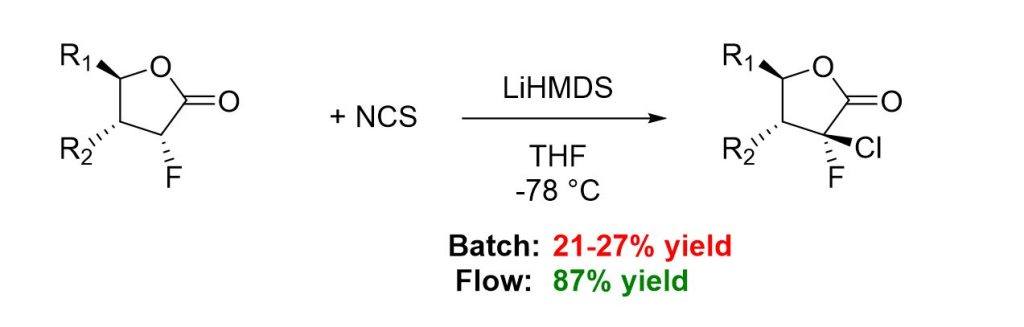
Scheme 1. Yield comparison of lactone chlorination in batch and by flow chemistry
Flow chemistry offered a robust alternative, allowing precise control over the residence time of the reaction mixture, independent of batch size. In our experimental setup (scheme 2 and picture 1), a first feed consisting of a solution of lactone and NCS in dry THF under nitrogen atmosphere was pumped and a second feed consisting of a solution of LiHMDS in dry THF under nitrogen atmosphere was pumped at the same flow rate. The two feeds were cooled to -78 °C then merged in a T-shaped mixing unit. The combined mixture was passed through a cooled reactor at -78°C for the appropriate residence time. After the reactor, the mixture was merged in a T-shaped mixing unit with a mixture of THF/AcOH allowing the quench of the reaction. The solution was then finally collected at room temperature. After optimization, side-product formation was efficiently suppressed by maintaining strict residence time control at low temperature followed by rapid quenching of the reaction stream. Furthermore, the straightforward scalability brought by flow chemistry enabled a productivity greater than 5g per hour. This approach ensured reproducible yields, with the desired product obtained in up to 87% yield, compared to only 21–27% under batch scale-up conditions, thereby establishing flow chemistry as a viable and scalable route for this transformation.
- Conclusion
Although the principles of flow chemistry date back to the early 20th century, its modern resurgence firmly established it as a key technology in chemical R&D. It enables rapid optimization, precise control, and reliable scalability—capabilities that are increasingly essential for efficient and sustainable chemical development. Far from being a simple alternative to batch processing, flow chemistry now represents a mature and versatile approach that supports safer operations, consistent quality, and smoother transitions from research to production. The integration of machine learning for predictive optimization, together with modular reactor platforms, will continue to strengthen the role of flow chemistry in pharmaceutical synthesis and beyond.
Call to action: Oxeltis invites you to explore the potential of flow chemistry for your specific applications. Our team is available to discuss how this technology can be integrated into your operations to deliver measurable benefits in efficiency, safety, and innovation. For more information on the subject or if you have any needs for flow chemistry, please contact us at contact@oxeltis.com
[1] Org. Process Res. Dev. 2016, 20, 2−25
[2] Org. Process Res. Dev. 2018, 22, 9, 1045–1062
[3] Acc. Chem. Res., 2019, 52, 2858−2869
[4] Catalysis Today, 2018, 308, 3-19
[5] Current Organic Chemistry, 2023, 27, 1090-1110
[6] Digital Discovery, 2025, 4, 2364–2400
[7] Chem.Soc.Rev.,2017, 46, 1250–1271
[8] Current Opinion in Green and Sustainable Chemistry 2024, 47:100907
[9] Angew. Chem. Int. Ed. 2021, 60, 8139 – 8148
[10] React. Chem. Eng., 2022,7, 1346-1357
[11] Org. Process Res. Dev. 2022, 26, 6, 1766–1793
[12] React. Chem. Eng., 2024,9, 2599-2609
[13] Chem. Eur. J. 2023, 29, e202203240
[14] Chem. Rev. 2017, 117, 18, 11796–11893

